Term 3 Second Entry: Diffusion and Osmosis
What is diffusion?
Diffusion is the net movement of particles from a region of higher concentration to lower concentration
Concentration refers to the number of particles per unit volume.
Diffusion will continue until the particles are uniformed distributed throughout the system. When this happens, the net movement of particles is zero and the equilibrium of the system is reached. Diffusion will stop.
Diffusion occurs spontaneously and does not involve any energy taken in or given out.
Gases diffuse the fastest as the gas molecules are spread apart from each other, thus moving the fastest, thus diffusion will occur fastest.
Osmosis
Here are some photographs of osmosis in a visking tube:
Osmosis is just a kind of diffusion: It is the net movement of WATER molecules from a region of higher concentration to lower concentration through a partially permeable membrane. These tiny holes located at the membrane is only large enough for water molecules to pass through, and none others. Like diffusion, osmosis onlky stops when the particles are distributed uniformly between the two solutions.
Types of solutions:
Isotonic Solution: Equal water concentration
Hypotonic Solution: Solution has higher concentration of water than the other solution
Hypertonic solution: Solution has lower concentration of water than the other solution.
Osmosis in living things
Osmosis is an important process for all living things because it is used in transporting food, waste and other substances in and out of the cells through the partially permeable membranes.
Respiration: When oxygen is used, the concentration of oxygen is lowered. When it is lowered, oxygen from the surroundings will diffuse into the cell through the cell membrane. Carbon dioxide is also produced, and it diffuses out from the cells to the surroundings which has a lower concentration of carbon dioxide.
Photosynthesis for green plants: Cells in the leaves use up carbon dioxide and produces oxygen. Carbon dioxide from the surrounding air diffuses into the cell of leaves and the oxygen diffuses out to the surrounding air.
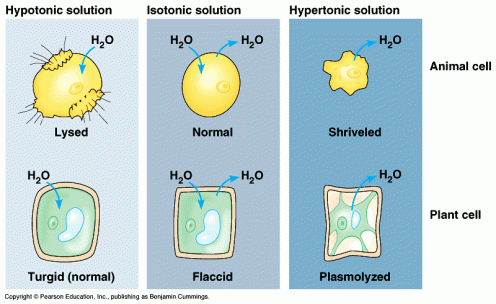
Animal cell in different types of solutions:
Hypotonic solution:
- Higher water concentration in extracellular solution than intracellular solution
- Water will enter the cell through osmosis
- Cell will eventually burst, as there is no cellulose cell wall to protect it, and only has a semi permeable cell membrane
- This results in a lysed cell
Hypertonic solution
- Lower water concentration in extracellular solution than intracellular solution
- Water exits from the cell via osmosis and it shrinks
- If large amount of water exits the cell, it becomes shrivelled
Isotonic solution
- Equal water concentration in both extracellular and intracellular solution
- No net gain or loss of water
- Animal cells prefer to be in this solution
Plant cell in different types of solutions
Hypotonic solution
- Higher water concentration in extracellular solution than in intracellular solution
- Net gain of water from extracellular solution into the cell
- Central vacuole increases in size and plant cell starts to swell
- However, they do not burst like animal cells due to its cell wall
- When the cell wall is stretched to the maximum, it is said to be turgid.
- Plant cells prefer to be in hypotonic solution
Hypertonic solution
- Lower concentration in extracellular solution than in intracellular solution
- Net gain of water into intracellular solution
- Central vacuole decreases in size before cell membrane starts to pull away from the cell wall
- Leaves a gap between cell wall and cell membrane. This process is called plasmolysis
- Plants with plasmolyzed cells will wilt and die due to lack of water
Isotonic solution
- Equal water concentration in extracellular solution than in intracellular solution
- No net gain or loss of water
- Cell appearance appearance is described as flaccid.